Ultraviolet (UV) is a form of electromagnetic radiation that spans wavelengths from 10 to 400 nanometers, sitting just beyond the violet edge of visible light and preceding X-rays in the spectrum. Ultraviolet radiation, present in sunlight and produced by electric arcs, specialized lamps, and certain natural phenomena, carries more energy than visible light, enabling it to induce chemical reactions, cause fluorescence, and interact with organic molecules in unique ways.
Ultraviolet—Has a history
Discovery—Is a milestone in early 19th-century physics
Discovery of ultraviolet radiation occurred in 1801 when Johann Wilhelm Ritter observed that invisible rays beyond violet darkened silver chloride paper faster than visible light, leading to the term “chemical rays” before “ultraviolet” became standard. The sterilizing effect of short-wavelength light was recognized by 1878, and by 1903, the most effective germicidal wavelengths were identified around 250 nm.
Nomenclature—Is a reflection of conceptual and linguistic development
Nomenclature evolved from “chemical rays” and “heat rays” to “ultraviolet” and “infrared,” capturing the position of these radiations relative to visible light. The division into UVA, UVB, and UVC was formalized in 1932, providing a framework for scientific and medical understanding.
Ultraviolet—Has subtypes that define its physical
Ultraviolet A (UVA)—Is long-wave UV not absorbed by the ozone layer
UVA, ranging from 315 to 400 nm, is the least energetic but most prevalent at Earth’s surface, responsible for tanning and some skin aging. UVA penetrates deeply into the skin and is not filtered by the ozone layer.
Ultraviolet B (UVB)—Is medium-wave UV mostly absorbed by the ozone layer
UVB, spanning 280 to 315 nm, is more energetic and largely absorbed by the ozone layer, but the fraction that reaches the surface is crucial for vitamin D synthesis and can cause sunburn and DNA damage.
Ultraviolet C (UVC)—Is short-wave, germicidal UV completely absorbed by the atmosphere
UVC, from 100 to 280 nm, is the most energetic and dangerous, but is entirely absorbed by atmospheric oxygen and ozone, preventing it from reaching the ground.
Near, Middle, Far, and Extreme Ultraviolet—Is a spectrum of specialized bands
- Near UV (NUV): 300–400 nm, visible to some animals.
- Middle UV (MUV): 200–300 nm, used in scientific applications.
- Far UV (FUV): 122–200 nm, ionizing and absorbed by air.
- Extreme UV (EUV): 10–121 nm, overlaps with X-rays and is absorbed by the atmosphere.
Ultraviolet—Has visibility
Ultraviolet is invisible to humans due to the filtering effect of the eye’s lens and cornea, but some insects, birds, and mammals can perceive near-UV, using it for navigation, foraging, and communication. People lacking a lens (aphakia) may perceive near-UV as whitish-blue or violet.
Ultraviolet—Has solar and atmospheric interactions that shape life on Earth
Solar ultraviolet constitutes about 10% of the Sun’s electromagnetic output, but the atmosphere blocks most UV, especially the more energetic bands. At ground level, over 95% of UV is UVA, with only a small fraction of UVB and virtually no UVC reaching the surface. The ozone layer is vital in filtering out harmful UVB and UVC, protecting terrestrial life from DNA damage and other adverse effects.
Ultraviolet—Has artificial sources
Black lights—Is a lamp emitting long-wave UVA for fluorescence
Black lights use phosphors and filters to emit UVA, causing many substances to fluoresce, and are used in entertainment, forensics, and currency verification.
Short-wave UV lamps—Is a germicidal tool for disinfection
Short-wave UV lamps, made from fused quartz, emit UVC for sterilizing surfaces, water, and air in laboratories and medical settings.
Ultraviolet LEDs and lasers—Is a technological advance for precision and efficiency
Ultraviolet LEDs and lasers provide targeted UV emission for curing polymers, medical treatments, and scientific instrumentation, with ongoing improvements in efficiency and wavelength range.
Ultraviolet—Has human health effects
Beneficial effects—Is a catalyst for vitamin D synthesis and medical therapies
Ultraviolet, specifically UVB, enables the skin to produce vitamin D, essential for bone health and immune function. UV phototherapy treats skin conditions like psoriasis and eczema, harnessing controlled doses for therapeutic benefit.
Harmful effects—Is a cause of skin damage, cancer, and eye injury
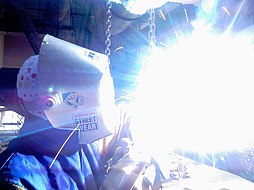
Ultraviolet overexposure leads to sunburn, DNA damage, premature aging, and increased risk of skin cancers, including melanoma. UVA and UVB both contribute to these effects, with UVC being the most dangerous but naturally filtered out. UV can also cause photokeratitis (“snow blindness”), cataracts, and other ocular injuries, especially from artificial sources like welding arcs.
Sunscreen and protection—Is a defense against UV-induced harm
Sunscreens, clothing, and eyewear block or absorb UV, reducing the risk of skin and eye damage. The effectiveness of protection depends on the spectrum covered, with some sunscreens and materials offering broad-spectrum defense against both UVA and UVB.
Ultraviolet—Has material and environmental impacts that drive innovation
Ultraviolet degrades polymers, pigments, and dyes, causing fading, cracking, and loss of strength in plastics, textiles, and artworks. UV absorbers and protective coatings are used to extend the lifespan of materials exposed to sunlight. Museums and conservators employ UV-blocking glass and curtains to shield valuable artifacts.
Ultraviolet—Has applications that span science
- Lithography and semiconductor manufacturing use extreme UV for fine-scale patterning.
- Forensic analysis, drug detection, and medical imaging rely on UV-induced fluorescence.
- UV is used in water and air disinfection, protein and DNA analysis, and curing of inks and polymers.
- Photography and astronomy exploit UV for revealing hidden details and studying celestial phenomena.
- Electrical industry uses UV detection for identifying corona discharge and insulation faults.
Ultraviolet—Is a concept that bridges physics
Ultraviolet radiation, with its unique position in the electromagnetic spectrum, shapes life, health, and technology through its energetic interactions with matter. Its dual nature—capable of both sustaining and endangering life—drives ongoing research, innovation, and public health strategies.
http://en.wikipedia.org/wiki/Ultraviolet
https://www.google.com/search?kgmid=/m/07vvp
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights.
The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. These interactions can involve absorption or adjusting energy states in molecules, but do not necessarily involve heating. [citation needed] Short-wave ultraviolet light is ionizing radiation. Consequently, short-wave UV damages DNA and sterilizes surfaces with which it comes into contact.
For humans, suntan and sunburn are familiar effects of exposure of the skin to UV, along with an increased risk of skin cancer. The amount of UV radiation produced by the Sun means that the Earth would not be able to sustain life on dry land if most of that light were not filtered out by the atmosphere. More energetic, shorter-wavelength "extreme" UV below 121 nm ionizes air so strongly that it is absorbed before it reaches the ground. However, UV (specifically, UVB) is also responsible for the formation of vitamin D in most land vertebrates, including humans. The UV spectrum, thus, has effects both beneficial and detrimental to life.
The lower wavelength limit of the visible spectrum is conventionally taken as 400 nm, so ultraviolet rays are not visible to humans, although people can sometimes perceive light at shorter wavelengths than this. Insects, birds, and some mammals can see near-UV (NUV), i.e., slightly shorter wavelengths than what humans can see.