Neoprene is a synthetic rubber created through the polymerization of chloroprene. It’s known for its chemical stability and flexibility across a wide range of temperatures. This versatile material finds its way into numerous products, from laptop sleeves to automotive fan belts.
The Birth of Neoprene
DuPont scientists invented neoprene on April 17, 1930. The breakthrough came after Elmer K. Bolton of DuPont attended a lecture by Fr Julius Arthur Nieuwland, a chemistry professor at the University of Notre Dame. Nieuwland’s work on acetylene chemistry led to the creation of divinyl acetylene, which formed an elastic compound when exposed to sulfur dichloride[1].
DuPont’s Wallace Carothers spearheaded the commercial development, collaborating with Nieuwland and other DuPont chemists. They refined the process, focusing on monovinyl acetylene’s reaction with hydrogen chloride gas to produce chloroprene[1].
Neoprene Evolves
DuPrene to Neoprene
DuPont initially marketed the compound as DuPrene in 1931. However, the original manufacturing process left an unpleasant odor. They developed a new method that eliminated this issue and cut production costs in half[1].
In 1937, DuPont replaced the DuPrene trademark with the generic name “neoprene.” This change aimed to position the material as an ingredient rather than a finished product. DuPont’s marketing strategy included publishing a technical journal to promote neoprene’s uses and advertise neoprene-based products from other companies[1].
Neoprene’s Unique Properties
Mechanical Marvels
Neoprene’s high tensile strength comes from its regular backbone structure, which allows it to undergo strain crystallization under tension. It’s tough stuff, with an ultimate tensile strength of 27.579 MPa and the ability to stretch up to 600% before breaking[1].
Environmental Resilience
This synthetic rubber outperforms natural rubber in resisting degradation. It’s got a higher burn point (around 260°C) than hydrocarbon-based rubbers, making it ideal for fire door weather stripping and combat gear[1].
Neoprene in Action
Aquatic Applications
Neoprene’s a star in the water. It’s the go-to material for wetsuits, drysuits, and fly fishing waders. The foamed version provides excellent insulation against cold water. However, divers should note that neoprene compresses under water pressure, offering less protection at greater depths[1].
Musical Contributions
The Rhodes piano switched to neoprene hammer tips around 1970, moving away from felt. You’ll also find neoprene in speaker cones and drum practice pads[1].
Pandemic Protection
During the COVID-19 pandemic, neoprene gained attention as an effective material for homemade face masks. Some commercial mask makers using neoprene claimed 99.9% filtration for particles as small as 0.1 microns[1].
Safety Considerations
While neoprene’s a versatile wonder material, it’s not without its drawbacks. Some folks are allergic to it, while others might develop dermatitis from thiourea residues left from production[1].
The vulcanization process often uses ethylene thiourea (ETU), classified as a reproductive toxin. From 2010 to 2013, the European rubber industry ran a research project called SafeRubber to find a safer alternative to ETU[1].
Neoprene’s story is one of scientific innovation meeting practical needs. From its accidental discovery to its widespread use today, it’s a material that continues to shape our world in countless ways.
Citations:
https://en.wikipedia.org/wiki/Neoprene
Neoprene (also polychloroprene) is a family of synthetic rubbers that are produced by polymerization of chloroprene. Neoprene exhibits good chemical stability and maintains flexibility over a wide temperature range. Neoprene is sold either as solid rubber or in latex form and is used in a wide variety of commercial applications, such as laptop sleeves, orthopaedic braces (wrist, knee, etc.), electrical insulation, medical gloves, liquid and sheet-applied elastomeric membranes or flashings, and automotive fan belts.
 A neck seal, wrist seal, manual vent, inflator, zip and fabric of a neoprene dry suit. The soft seal material at the neck and wrists is made from single backed closed-cell foam neoprene for elasticity. The slick unbacked side seals against the skin. The blue area is double-backed with knit nylon fabric laminated onto closed cell foamed neoprene for toughness. Some insulation is provided by the suit, and the rest by garments worn underneath. | |
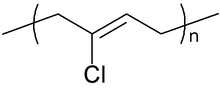 Chemical structure of the repeating unit of polychloroprene | |
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.127.980 |
| EC Number |
|
CompTox Dashboard (EPA) | |
| Properties | |
| Density | 1.23 g/cm3 (solid) 0.1-0.3 g/cm3 (foam) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
English
Etymology
US, 1930s; genericized trademark for DuPont brand of polychloroprene. neo- (“new”
...
